Case Report
Retrospective Descriptive Study on Non-operative Management of Epidural Haematoma in a Cohort of Patients at the Korle Bu Teaching Hospital
Thomas K Dakurah1*, Hadi Mohammed Abdullah2, Fuseini Adams2, Emmanuel Bannerman- Williams3 and Benjamin Abaidoo4
1Department of Surgery, Neuroscience Unit, School of Medicine and Dentistry, College of Health Sciences,
University of Ghana, Ghana
2Department of Surgery, Neuroscience Unit, Korle Bu Teaching Hospital, Ghana
3Department of Surgery, School of Medicine and Dentistry, College of Health Sciences, University of Ghana,
Ghana
4Department of Surgery, Ophthalmology Unit, School of Medicine and Dentistry, College of Health Sciences,
University of Ghana
*Corresponding author: Thomas K Dakurah, Department of Surgery, Neuroscience Unit, School of Medicine and Dentistry, College of Health Sciences, University of Ghana
Published: 06 Dec, 2017
Cite this article as: Dakurah TK, Abdullah HM, Adams F,
Bannerman-Williams E, Abaidoo B.
Retrospective Descriptive Study on
Non-operative Management of Epidural
Haematoma in a Cohort of Patients at
the Korle Bu Teaching Hospital. Clin
Surg. 2017; 2: 1802.
Abstract
Background: Head injury resulting from trauma is the main cause of death in people younger than
45 years. Extradural hematoma is the most serious treatable complication of head injury, requiring
immediate diagnosis and surgical intervention.
Objective: To determine whether selected cases of Acute Epidural Haematoma can be managed
non-operatively.
Methods: This is a retrospective study of acute epidural haematomas managed non-operatively
from a cohort of 107 cases at the Korle-Bu Teaching Hospital from 7th November, 2012 to 15th
February, 2016. Patients’ demographic and clinical information including case notes and CT scan
findings were retrieved and analyzed with IBM SPSS version 20. Total cost of hospital bills including
the cost of medication, CT scans and hospitalization of patients managed non-operatively were
compared to those managed operatively.
Results: A total of 19 cases of EDH were managed conservatively over the study period. There was
a male preponderance of 78.95%. The mean age was 30.89 years with mean duration (in days) of
hospital stay as 10.42 days. Motor vehicular accident (MVA) was the main mechanism of injury
(55.56%). A single mortality was recorded at 3 months post-discharge. The total estimated cost
of craniotomy and evacuation of haematoma was 4 times (75% higher) that of non-operative
management.
Conclusion: It has become increasingly apparent that many small epidural hematomas will resolve
with nonsurgical management without neurologic sequelae. This study also showed that nonoperative
management of acute epidural haematoma may be cost-effective compared with operative
management.
Keywords: Epidural haematoma; Non-operative management; Tempo-parietal; Neurological
deficit; Glasgow Coma Scale; Middle income country
Introduction
Trauma is the leading cause of death in people younger than 45 years and head injury is the
main cause of trauma mortality [1,2]. Epidural haematoma (EDH) refers to the occurrence of
symptomatic bleeding within the potential space between the skull and the dura. EDH accounts for
1% of all head injuries and 5% to 15% of severe traumatic brain injuries [3,4].
EDH has a male to female ratio of 4 to 1 and remains uncommon among children under 2
years old as a result of the plasticity of the skull and beyond 60 years on account of the adherence
of the dura to the inner table. The imaging standard for the diagnosis of EDH is the Computed
Tomography (CT) scan [5,6]. Before the CT scan age, cerebral angiography, exploratory burrhole
and pneumo-encephalography were the main diagnostic approaches for managing patients with EDH. The standard treatment involved urgent evacuation of
the haematoma via a craniotomy with minimal invasive procedures
advocated to prevent brainstem compression or decompensation
[7,8].
EDH remains an important consequence of acute head injury [9].
Notwithstanding, there are reported cases of successful management
of some cases non-surgically [10-12]. The percentage of patients
managed non-operatively in clinical series since the 1960s has
progressively increased from less than 1% to more than 60% [12].
Other studies have also reported few cases of conservatively managed
EDHs requiring surgical conversion as a result of deteriorating
neurological status [13,14]. EDH patients must be admitted in a
high dependency unit with careful monitoring. Such services are
associated with high cost. Unfortunately many centers in Africa lack
these facilities and services. Neurosurgical services in Ghana and
other developing countries are still evolving, as such, there tend to
be an unacceptable delay before appropriate referral of patients to a
competent centre. Even when cases arrive at such a centre there may
still be undue delays because of institutional and logistical challenges.
Major operational problems encountered at the Neuroscience Unit
at the Korle Bu Teaching Hospital (KBTH) includes inadequate
operating room space and time, lack of appropriate supporting staff
(operating room, critical care and specialized neuroscience nurses),
inadequate anaesthetic support and equipment. This usually results
in delay in accessing appropriate care including surgical intervention.
There is the need therefore for non-operative management of some
cases of acute EDH, when carefully selected. This research aims at
studying the outcomes of conservative management of selected cases
of acute EDH in a resource-constrained environment.
Table 1
Materials and Methods
This is a retrospective cross-sectional study of acute EDH
managed non-operatively from a cohort of 107 consecutive cases at
the Neuroscience Unit of the Korle-Bu Teaching Hospital within the
period 7th November, 2012 to 15th February, 2016.
Included in this study were patients diagnosed with acute
EDH with the absence of neurological deficit corresponding to
the haematoma, a Glasgow Coma Scale (GCS) greater than 9, a
haematoma thickness less than 15 mm, a haematoma volume less
than 30 mls and a midline shift less than 5 mm. However, patients
diagnosed with acute EDH with the presence of neurological deficit,
a GCS less than 9, a haematoma thickness greater than 15 mm, a
haematoma volume greater than 30 mls, a midline shift greater than
5 mm and a location of the haematoma at the temporal area and
posterior cranial fossa were excluded from this study. The case notes
of all the 107 consecutive patients with a CT scan confirming acute
EDH admitted to the neuroscience unit between November 2012 and
February 2016 were retrieved and reviewed. Out of these 107 patients,
those who were initially managed non-operatively had their case notes
and CT scans further evaluated. A review of the initial and followup
CT scans was done and lesion diameter, volume, midline shift
and location of the haematoma were determined and documented.
Other intracranial injuries including skull fractures were also noted.
Measurements were made with calipers and a measuring scale (mm)
devised from the scale printed on the CT images. The volume of the
haematoma (EDHV) was calculated using the Peterson and Esperson
equation: (a × b × c × 0.5), where a, b and c are the widest diameters
of the haematoma in the axial, sagittal and corona planes respectively.
The age, sex, and duration of hospital admission were also
recorded. The Glasgow Outcome Score (GOS) by Jennett & Bonds
was used to determine the outcome of each patient managed nonoperatively
at discharge and 6 weeks, 3 months and 6 months post
discharge respectively [15].
The outcomes included; death, vegetative state, severe disability,
moderate disability and good recovery respectively. A good outcome
measure was defined as having a moderate disability or good recovery.
Poor outcomes were those who died, including those with vegetative
state and severe disability.
The total hospital bill and cost of initial and follow up CT scans
were determined using records from the account units of the Surgical
and Radiology Departments of the Korle Bu Teaching Hospital.
These were compared with bills of patients managed surgically which
includes; operating room fees, peri-operative medications including
anaesthesia, intensive care, and the average cost of transportation
to and from hospital for wound inspection and dressing and postdischarge.
There was no voluntarily written informed consent since the
study was retrospective. However, the principles in human subject
protection in accordance with the Helsinki Declaration in Medical
Research were upheld.
Data was entered on a Microsoft Excel version 2013 spreadsheet
with analysis performed using IBM SPSS version 20 software. Clinical
and background information of study participants were analyzed
and presented descriptively as means (standard deviations) for
continuous variables and counts and proportions for categorical
variables. Mechanism of injury, site of haematoma, skull fracture
and craniofacial injuries were also analyzed and presented as counts
and proportions. The mean outcome score at discharge, 6 weeks, 3
months and 6 months intervals respectively were computed.
The Independent Samples T-Tests at 95% confidence interval was
used to compare the mean difference in cost between non-operative
and operative management of acute EDH. P-values less than 0.05 were taken as statistically significant (Table 1).
Table 2
Table 3
Results and Discussion
The study recorded a total of 19 patients with a male preponderance
(78.95%) and an average age of 30.89 years from Table 1. Most
(84.21%) patients were aged 16-64 years. Patients were brought to the
hospital an average of two days (std. dev. of 3.43 days) after injury.
The mean duration of hospital admission (stay) recorded was 10.42
days (std. dev. of 5.38 days). Most (84.21%) patients were transferred
from other health facilities to the KBTH. Out of 16 cases for which
data on clinical signs at presentation was available, headache was the
main (68.75%) presenting clinical symptom (Table 2). From Table
2 above; the main mechanism of injury was motor vehicle accident
which occurred among 10 patients (52.63%). Pedestrian accident
(PA), assault, fall and others (work related and a struck by a fallen
television set) accounted for two cases each respectively with motor
bike accident responsible for a single case (Table 3,4).
From Table 4 above, 42.11% of the patients reported with
scalp laceration and 21.05% with facial abrasions. Subconjunctival
haemorrhage was reported in 15.80% of the patients with 5.26%
each for lip lacerations, facial lacerations, multiple abrasions and
periorbital edema respectively (Table 5). About 95% of the patients
had a GOS of 4 or above. This is a good outcome. One patient had
an Outcome score of 1 (Table 6). The average cost of non-operative
management was GHC 811.25 ($200). This includes the cost of CT
scans, medication and hospitalization. A second CT scan was required
if the GCS dropped by 2 or more points, when patient developed a
lateralizing sign attributable to the haematoma and the presence of
persistent headaches. The non-operatively managed patient required
an average of 3 CT scans (admission, discharge and 3 months followup).
Those who had surgical intervention required an average of 2
CT scans (admission and 3 months follow-up. The average cost of
operative management was GHC 3277.89 ($819). This was inclusive
of fees for CT scans, cost of medication, surgery, intensive care unit
for the first 24 hr. The difference between the two modalities was
significant at a p-value of 0.0001 (this is an estimated cost) (Figure
1,2).
Acute epidural haematoma is a relatively uncommon presentation
in neurosurgery. It accounts for between 1% to 5% of all head injuries,
and even higher in severe head injuries (15%) [3,4].
Trauma with involvement of the middle meningeal artery is
the common cause [15]. Haematoma in the temporal fossa may be
associated with rapid deterioration due to uncal herniation. This has
been observed even with smaller volumes of haematoma in contrast to
other locations [16]. The classical presentation of EDH is one of brief
loss of consciousness followed by a lucid interval. However in the era
of CT scan it has been found that many patients with acute EDH do
not present classically [17,18]. Prior to the invention of the CT scan,
there had been a reported case of chronic epidural haematoma which
was managed conservatively [19]. With the advent of CT scan, 29
publications on conservatively managed acute EDH cases have been
reported between 1981 and 2014 [20].
Some cases of acute EDH managed conservatively required
subsequent surgical intervention as a result of neurological
deterioration; 13% of cases in a study by Knuckey et al. [21] required
surgical conversion.
This study shows a male preponderance of 78.95% which is
similar to the findings of Sullivan et al. [22] for cases managed nonoperatively.
The mean age of 30.89 years is in keeping with other
studies on acute EDH in the West African sub region [4]. Ndoumbe et
al. [10] reported that 63% of cases of EDH in their centre in Cameroun
were between 29 to 30 years. In that study, 93.4% were males.
This male preponderance can be attributed to the socioeconomic
practices of the population of this region in which majority of females
are housewives, thus leaving the males with the responsibility of engaging in highly physical activities. The 2013 WHO global report
on road traffic injury prevention shows that the younger age group
predominate road traffic accidents in low income countries [23]. This
is however different in high income countries where about 20% of
drivers are more than 65 years old [23].
Three of the patients were below 15 years with one being 2
years. This is consistent with other studies which show the rarity of
EDH in children less than 2 years [24,25]. Children below 10 years
accounted for 11.8% of cases of EDH reported by Milan et al. [26] this
is comparable to our finding of 15.8%.
Majority of Patients in this study were transferred to the accident
centre of the Korle-Bu Teaching Hospital two days after the injury.
These patients thus reported more than 6 hours after the injury.
Weaver et al. [27] reported 2 cases of EDH. Of these two, one had
a temporal EDH with a CT scan performed 16 hr after injury, and
the other with a temporoparietal extradural haematoma diagnosed
3 days after injury. This is suggestive of successful conservative care
in this group of patients who present late with high coma scores.
Patients who had CT scans done less than 6 hr of trauma on account
of coma were found to have haematoma volume of more than 30 mls
by Bullock et al. [28] Such patients did not do well with conservative
management and needed surgical evacuation. This was not the case
with patients in this study. In this current study most patients were
brought in 2 days after injury with a GCS of 14 and minimal or no
focal deficits.
The criteria for managing EDH non-operatively vary in the
literature. Whiles others considered mainly the neurological status,
Bullock, Lee and Servadei chose CT scan features [28-30]. Bullock et
al. [12] in 1985 reported 22 cases of EDH (12 to 38 ml in volume) which
were all managed conser-vatively and with a complete reabsorption of
the haematoma. Another study by Bullock et al. [28] in 2006 reported
and strongly recommended urgent surgical management for patients
in coma (GCS score < 9) with anisocoria and for those with blood clot
larger than 30 cm3 in size, regardless of the patient’s Glasgow Coma
Scale (GCS) score. Those patients with EDH less than 30 cm3, less
than 15-mm in thickness, with less than 5-mm of midline shift, and
GCS score greater than 8 without focal deficits were regarded safe
for nonsurgical management with close observation and serial CT
scans [28]. Servadei et al. [30] also used a haematoma thickness of 1.0
cm and a midline shift of less than 0.5cm irrespective of location as
criteria for non-operative management. Thus, the CT scan inclusion
criteria in this study were reasonable. Although there are reported
cases of EDH in locations such as the temporal and posterior fossa
managed non-operatively, haematomas in these regions have call for
closer monitoring [31,32]. This study did not include haematomas in
the temporal and posterior fossa because of limited ICU/HDU space
at the study centre for close monitoring. Lesions in these regions can
deteriorate fast [33].
Patients in this study were admitted to the ward and put on a
neurological flow chart and four hourly observations in the GCS,
blood pressure, pulse and respiratory rate. The patients were also
monitored for lateralizing signs. A reduction in GCS by 2 points or
more necessitated a new CT scan to assess the haematoma. GCS below
9 has been found to be associated with poor outcome irrespective of
the method of management [34]. In the study by Tantanaru et al.
[35], 28 patients underwent urgent surgery. Thirteen patients were
managed non-operatively and 15 patients required delayed surgery
because of neurological deterioration or increase of the size of the
hematoma.
They suggested that haematoma volume of 30 mls or more is
an indication for conversion to surgery. Two of the patients in their
study had GCS below 9 and both of them had poor outcomes. Bhau
et al. [36] reported that, out of the 120 patients managed for EDH,
67 were managed conservatively and 31 had immediate surgical
intervention. Twenty two of their study participants had delayed
surgery and the main reason for conversion was neurodeterioration
though majority had haematoma volume of >25 mls. In this current
study, the mean GCS of patients at admission was 14, even though the
inclusion criterion was 9 to 15.
Majority of the patients were on admission for an average period
of 10 days. This is similar to other reports of conservatively managed
epidural haematomas [5,37]. Balmer et al. [37] reported 13 cases of
epidural haematoma which were managed conservatively with an
average duration of admission of 9.6 days. Hamilton et al. [5] reported
an average admission period of 11.2 days for 18 patients in their series
who were managed non-operatively and an average of 27.6 days for
30 patients who were surgically managed from the onset.
The main presenting complaint was headache of varying intensity.
Only one patient out of the 19 had a seizure. Of the 11 patients who
had headaches, only one required surgical intervention on account
of its persistence. A repeat CT scan showed an increase in size of the
haematoma with decrease in density. Burr-hole and drainage was done for him a week after admission resolving the headache.
The presence of an associated skull fracture is reported to have a
positive influence on the resolution of EDH. It has been shown that
there is epicranial transfer of the blood in the presence of an overlying
fracture [38]. Tuncer et al. [38] reported associated skull fracture in
70% of patients with EDH which were managed conservatively. This
current study recorded skull fractures in 56% of the patients seen.
Chen et al. [18] and Ahmad et al. [39] also found that skull fracture
was not a positive predictor of progressive epidural haematoma. Skull
fracture had been considered a risk factor for haematoma progression
in earlier studies, but subsequent reports have shown different results
[40].
In a study by Arvind et al. [41], the GCS at presentation was
identified as the single most important predictor of good outcome in
patients managed conservatively. They enrolled patients with GCS of
13 or better. However, Bullock et al. [12], Lee et al. [29], Servadeial et
al. [30] and Lobato et al. [40] considered both volume and neurological
status as the factors which influence outcomes. The mean GCS of
our patients was 14 or better and all patients were discharged home
with GCS of 15. Only one patient needed surgical intervention for
persistent headache with some increase in the haematoma size over
time. The GCS of this patient remained 15 throughout the admission
period.
One patient died 3 months into follow-up, post discharge. He had
shown good recovery clinically and radiologically. His GCS remained
15 throughout admission and at 3 months follow-up. He died in a
primary healthcare facility close to his home and the cause of death
was not ascertained.
Balmer et al. [37] reported no adverse finding in children with
acute EDH they managed conservatively. These children were
followed-up for 8 years. In another series involving 65 cases, more
than 50% were managed non-operatively [42]. All the patients were 16
years and below. Six cases required surgical intervention on account
of deterioration with a corresponding increase in haematoma size on
CT done within 48 hr of admission. Khan et al. [43] also reported
a series of 17 pediatric cases in which 2 patients’ required surgical
conversion. The patients had GOS score of 5 and normal schooling
at 1 year of follow-up. Cayli et al. [44] used single photon emission
computed tomography (SPECT) scan to follow up patients for 3 and
6 months and reported no pathological findings.
The management of EDH is based on both clinical and radiological
findings. Increasingly, the percentage of cases managed conservatively
has progressed from 1% to 60% since the 1960s [29-31]. Regular or
scheduled examination of the patient for neurological deterioration
has been reported to be the most important factor which determines
the need for repeat CT scan and subsequently surgical intervention.
Sullivan et al. [22] reported that haematomas that will increase in
size achieve that within the first 36 hr. However this increase was not
associated with deterioration in neurological status. We recorded
only one haematoma expansion amongst the research participants.
Neuroimaging must therefore be repeated only if the need arises. In a
resource constraint environment such as ours, this is relevant. Repeat
CT scans were done only when the neurological state necessitated
it. On the average 2 CT scans were required prior to discharge. In
the more affluent world, this will be unacceptable. The emphasis has
shifted from ‘wait and see’ to ‘diagnose and treat’ especially in cases
of mild head injuries.
Cases managed operatively in our institution, paid for anaesthesia,
surgery and ICU services in addition to the normal ward bills and cost
of CT scans. With the composite billing system employed, it implies
surgery attracted huge costs. Craniotomy is designated as a ‘major
complex’ surgery by the coding system. Conservative management
attracted the usual ward bills, medications and cost of CT scans. This
was 4 times less than the cost for operative management. Even though
the cost estimates made, may not reflect the entire healthcare costs of
treating these patients, to the best of our knowledge this is the first
attempt in our environment.
Conclusion
Despite the risk of an untreated EDH, our study adds to the body of knowledge that carefully selected cases can be managed nonoperatively. Neurological monitoring remains the most important factor in determining patient deterioration and not neuroimaging. When properly done, it reduces the number of repeat CT scans required to evaluate progress. The study also showed that nonoperative management may be cost-effective in a resource-constrained setting. A prospective study on the subject will further enhance the knowledge and reduce unnecessary surgical interventions.
Figure 1
Figure 1
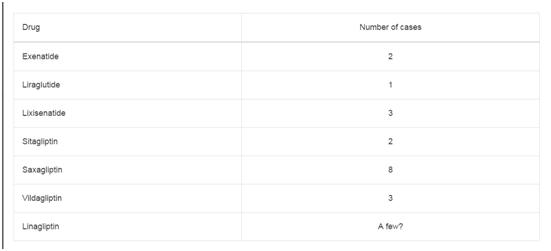
Patient A: A 51 year old female who presented with headache
and a GCS of 15 following Road traffic accident. She was managed
conservatively and discharged home on day 9. A repeat scan at 3 months
showed a complete resolution of the haematoma.
A1: CT scan of Patient A on the first day of admission showing haematoma.
A2: CT scan of Patient A after a week of admission showing a resolving
haematoma.
A3: A repeat CT scan of Patient A after 3 months showing a complete
resolution of haematoma.
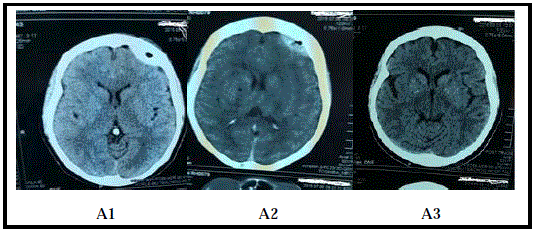
Figure 2
Figure 2
Patient B: A 51 year old male who presented with restlessness
and a GCS of 14/15. He was managed conservatively and discharged home
after 12 days of admission. His GCS at discharge was 15/15.
B1: CT scan of Patient B on the first day of admission showing haematoma.
B2: CT scan of Patient B after 10 days of admission showing an almost
resolved haematoma.
Table 4
Table 5
Table 6
Table 6
Cost effectiveness of conservative management of subjects with EDH at
the Korle Bu Teaching Hospital
Acknowledgement
Our acknowledgement goes to all the staff of the Neuroscience unit, Korle-Bu Teaching Hospital.
References
- MacKenzie EJ. Epidemiology of injuries: current trends and future challenges. Epidemiol Rev. 2000;22(1):112-9.
- Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21(5):375-8.
- Jamieson KG, Yelland JD. Extradural hematoma. Report of 167 cases. J Neurosurg. 1968;29(1):13-23.
- Mezue WC, Ndubuisi CA, Chikani MC, Achebe DS, Ohaegbulam SC. Traumatic extradural hematoma in enugu, Nigeria. Niger J Surg. 2012;18(2):80-4.
- Hamilton M, Wallace C. Nonoperative management of acute epidural hematoma diagnosed by CT: the neuroradiologist's role. AJNR Am J Neuroradiol. 1992;13(3):853-9.
- Marshall LF. Head injury: recent past, present, and future. Neurosurgery. 2000;47(3):546-61.
- Rehman L, Khattak A, Naseer A, Mushtaq. Outcome of acute traumatic extradural hematoma. J Coll Physicians Surg Pak. 2008;18(12):759-62.
- Huang AP, Huang SJ, Hong WC, Chen CM, Kuo LT, Chen YS, et al. Minimally invasive surgery for acute noncomplicated epidural hematoma: an innovative endoscopic-assisted method. J Trauma Acute Care Surg. 2012;73(3):774–7.
- Tallon JM, Ackroyd-Stolarz S, Karim SA, Clarke DB. The epidemiology of surgically treated acute subdural and epidural hematomas in patients with head injuries: a population-based study. Can J Surg. 2008;51(5):339-45.
- Ndoumbe A, Ekeme MVP, Jemea B, Simeu C, Takongmo S. Epidemiological Analysis of Surgically Treated Acute Traumatic Epidural Hematoma. OJMN. 2016;06(03):89-97.
- Hooper R. Observations on extradural haemorrhage. Br J Surg. 1959;47:71-87.
- Bullock R, Smith RM, van Dellen JR. Nonoperative management of extradural hematoma. Neurosurgery. 1985;16(5):602-6.
- Pozzati E, Tognetti F. Spontaneous healing of acute extradural hematomas: study of twenty-two cases. Neurosurgery. 1986;18(6):696-700.
- Brodin H. Extradural hematomas. A survey of cases covering a 20 year period with special reference to diagnosis. Acta Chir Scand. 1951;102(2):99-109.
- Ford LE, Mclaurin RL. Mechanisms of Extradural Hematomas. J Neurosurg. 1963;20:760-9.
- Illingworth R, Shawdon H. Conservative management of intracranial extradural haematoma presenting late. J Neurol Neurosurg Psychiatry. 1983;46(6):558-60.
- Flannery AM. Cautions in the conservative management of epidural hematomas. Pediatr Neurosurg. 2009;45(3):185.
- Chen H, Guo Y, Chen SW, Wang G, Cao HL, Chen J, et al. Progressive Epidural Hematoma in Patients with Head Trauma: Incidence, Outcome, and Risk Factors. Emerg Med Int. 2012;2012:134905.
- Tochio H, Waga S, Tashiro H, Takeuchi T, Miyazaki M. Spontaneous resolution of chronic epidural hematomas: report of three cases. Neurosurgery. 1984;15(1):96-100.
- Maugeri R, Anderson DG, Graziano F, Meccio F, Visocchi M, Iacopino DG. Conservative vs. Surgical Management of Post-Traumatic Epidural Hematoma: A Case and Review of Literature. Am J Case Rep. 2015;16:811-7.
- Knuckey NW, Gelbard S, Epstein MH. The management of "asymptomatic" epidural hematomas. A prospective study. J Neurosurg. 1989;70(3):392-6.
- Sullivan TP, Jarvik JG, Cohen WA. Follow-up of conservatively managed epidural hematomas: implications for timing of repeat CT. AJNR Am J Neuroradiol. 1999;20(1):107-13.
- World Health Organization. World report on road traffic injury prevention. 2004.
- Irie F, Le Brocque R, Kenardy J, Bellamy N, Tetsworth K, Pollard C. Epidemiology of traumatic epidural hematoma in young age. J Trauma. 2011;71(4):847-53.
- Jamous MA, Abdel Aziz H, Al Kaisy F, Eloqayli H, Azab M, Al-Jarrah M. Conservative management of acute epidural hematoma in a pediatric age group. Pediatr Neurosurg. 2009;45(3):181-4.
- ul Haq MI. Traumatic extradural Haematoma. Professional Med J. 2004;21(3):540-3.
- Weaver D, Pobereskin L, Jane JA. Spontaneous resolution of epidural hematomas. Report of two cases. J Neurosurg. 1981;54(2):248-51.
- Bullock MR, Chesnut R, Ghajar J, Gordon D, Hartl R, Newell DW, et al. Surgical management of acute epi¬dural hematomas. Neurosurgery. 2006;58:S7–15.
- Lee EJ, Hung YC, Wang LC, Chung KC, Chen HH. Factors influencing the functional outcome of patients with acute epidural hematomas: analysis of 200 patients undergoing surgery. J Trauma 1998;45:946-52.
- Servadei F, Faccani G, Roccella P, Seracchioli A, Godano U, Ghadirpour R, et al. Asymptomatic extradural haematomas. Results of a multicenter study of 158 cases in minor head injury. Acta Neurochir (Wien). 1989;96(1-2):39-45.
- Bor-Seng-Shu E, Aguiar PH, de Almeida Leme RJ, Mandel M, Andrade AF, Marino R Jr. Epidural hematomas of the posterior cranial fossa. Neurosurg Focus. 2004;16(2):ECP1.
- Kang SH, Chung YG, Lee HK. Rapid disappearance of acute posterior fossa epidural hematoma. Neurol Med Chir (Tokyo). 2005;45(9):462-3.
- Shah MV. Conservative management of epidural hematomas: is it safe and is it cost-effective? AJNR Am J Neuroradiol. 1999;20(1):115-6.
- Seelig JM, Marshall LF, Toutant SM, Toole BM, Klauber MR, Bowers SA, et al. Traumatic acute EDH, unrecognized lethality in comatosed patients. Neurosurg.1984;15:617–20.
- Tataranu L, Ciubotaru V, Paunescu D, Spatariu A, Radoi M. Extradural hematoma – is surgery always mandatory? Rom J Leg Med. 2014;22:45-50.
- Bhau KS, Bhau SS, Dhar S, Kachroo SL, Babu ML, Chrungoo RK. Traumatic extradural hematoma - role of non-surgical management and reasons for conversion. Indian J Surg. 2010;72(2):124-9.
- Balmer B, Bolthauser E, Altermatt S, Gobet R. Management of significant epidural haematoma in children. Childs NervSyst. 2006;22:363-67.
- Tuncer R, Kazan S, Uçar T, Açikbas C, Saveren M. Conservative management of epidural haematomas. Prospective study of 15 cases. Acta Neurochir (Wien). 1993;121(1-2):48-52.
- Ahmad T, Imran S, Sarfraz K. Risk factors of progressive epidural hematoma in patients with head trauma. RMJ. 2015;40:303-6.
- Lobato RD, Rivas JJ, Cordobes F, Alted E, Perez C, Sarabia R, et al. Acute epidural hematoma: An analysis of factors influencing the outcome of patients undergoing surgery in coma. J Neurosurg 1988;68:48-57.
- Dubey A, Pillai SV, Kolluri SVR. Does volume of extradural haematoma influence management strategy and outcome? Neurol India. 2004;52(4):443-5.
- Ilie G, Boak A, Adlaf EM, Asbridge M, Cusimano MD. Prevalence and correlates of traumatic brain injuries among adolescents. JAMA. 2013;309(24):2550-2.
- Khan MB, Riaz M, Javed G. Conservative management of significant supratentorial epidural hematomas in pediatric patients. Childs Nerv Syst. 2014;30(7):1249-53.
- Cayli S, Beskonakli E, Bestepe E, Okay O, Nalkoden S, Taskin Y. Asymptomatic or minimally symptomatic traumatic epidural haematomas: Comparison of the results of surgical and conservative management related to SPECT and neuropschological tests. Preliminary. Neurosurg Rev. 1998;21:226-31.